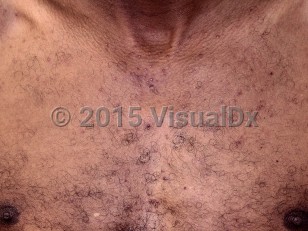
Acneiform eruptions are seen in 75%-90% of EGFRi-treated patients, but the incidence and severity vary with the type of drug used, being higher with the monoclonal antibodies cetuximab and panitumumab than with the low molecular weight tyrosine kinase receptor inhibitors erlotinib, lapatinib, and gefitinib. Lapatinib, being an EGFR / human epidermal growth factor receptor 2 (HER2) inhibitor, has a much lower incidence of side effects.
The EGFR plays a vital role in epidermal homeostasis by regulating keratinocyte proliferation, differentiation, and survival. Inhibition of the EGFR leads to the elaboration of several inflammatory cytokines and chemokines, and recruitment of inflammatory cells (neutrophils and lymphocytes) in the dermis, ultimately resulting in a papulopustular eruption.
Onset is within the first 2 weeks of initiation of treatment (range 3-182 days) and usually resolves within 4 weeks of discontinuation of EGFRi therapy.
The eruption typically appears on the face, scalp, and upper trunk and may be associated with pain, burning, pruritus, and dysesthesias.
Increased incidence and severity of acneiform eruptions have been associated with higher doses of the drug and sun exposure (ultraviolet [UV] radiation). Onset of rash has been associated with positive treatment response to EGFRi.
MEK inhibitors (MEKi) such as trametinib, selumetinib, cobimetinib, and binimetinib cause a similar papulopustular eruption as EGFRi and can typically be managed similarly to the EGFRi papulopustular eruption.
Four distinct clinical phases of evolution may be noted:
- Week 1 – sensory disturbance (erythema, edema, and dysesthesias)
- Weeks 1-3 – appearance of papulopustular eruption
- Weeks 3-4 – crusting (drying of purulent material)
- After 4 weeks – residual erythema, telangiectasias, and xerosis
- Grade 1 – Papules and/or pustules covering less than 10% body surface area (BSA), which may or may not be associated with symptoms of pruritus or tenderness.
- Grade 2 – Papules and/or pustules covering 10%-30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; associated with psychosocial impact; limiting instrumental activities of daily living (ADL).
- Grade 3 – Papules and/or pustules covering more than 30% BSA, which may or may not be associated with symptoms of pruritus or tenderness; limiting self-care ADL; associated with local superinfection, with oral antibiotics indicated.
- Grade 4 – Papules and/or pustules covering more than 30% BSA, which may or may not be associated with symptoms of pruritus or tenderness and are associated with extensive superinfection, with intravenous (IV) antibiotics indicated.
- Grade 5 – Death.
EGFRi therapy has been associated with other cutaneous side effects, including xerosis and asteatotic dermatitis, nail changes (paronychia and pyogenic granulomas), fissuring of the fingertips, hair changes (trichomegaly, brittle hair, follicular inflammation, inflammatory alopecia, and hair shaft abnormalities such as pili torti), mucositis and stomatitis, and photosensitivity.