Sarcoidosis commonly presents with abnormalities identified incidentally on chest radiography. Although the disease can affect different organs, systemic symptoms such as fever, night sweats, and weight loss are common. Sarcoidosis can affect the lungs, with symptoms such as chest pain, shortness of breath, cough, and most commonly, pronounced fatigue. It can also affect the peripheral lymph nodes, heart, kidneys, gastrointestinal tract, central nervous system (CNS), liver, spleen, bone, muscle, and endocrine glands. Venous thromboembolism and pulmonary hypertension are potential complications of sarcoidosis. Approximately 90% of patients will have lung involvement. Pulmonary fibrosis and bronchiolectasis result in "honeycombing" of the lung and represent end-stage lung disease due to chronic granulomatous inflammation. Hilar lymphadenopathy is asymptomatic and affects 90% of patients. Approximately 10% of patients have hypercalcemia.
Granulomas of the eyelids, lacrimal glands, conjunctiva, iris, retina, and choroid can occur. Rarely, there can be granulomatous inflammation of the extraocular muscles leading to diplopia, which may be the presenting sign of sarcoidosis.
Ten to thirty percent of patients will present with Löfgren syndrome with arthritis, erythema nodosum, and bilateral hilar adenopathy. Women more commonly have erythema nodosum, and men more commonly have ankle periarticular inflammation or arthritis. Two-thirds of patients achieve remission within a decade with few consequences, with the majority within 3 years. The remaining one-third have progressive disease with significant organ impairment.
Neurosarcoidosis commonly manifests as cranial-nerve deficits. Any part of the neurologic system can be impacted by sarcoidosis; a vast range of neurologic signs and symptoms may be present. Delays in diagnosis and treatment can lead to permanent disability. The diagnostic workup for neurosarcoidosis should include an MRI of the head, cerebrospinal fluid analysis, and detection of sarcoidosis outside the nervous system.
Approximately 25% of patients will have cutaneous involvement, and some patients may have skin-limited disease. Asymptomatic red-brown dermal papules and/or plaques that favor the face, neck, upper extremities, and upper trunk are the most common specific cutaneous sarcoid lesions. There are several other morphologic variants of cutaneous sarcoidosis:
- Lupus pernio – Violaceous papules and plaques that are most commonly found on areas affected by the cold (hence, the name pernio), such as the nose, ears, and cheeks. In contrast to other cutaneous sarcoid lesions, lupus pernio can result in scarring.
- Verrucous lupus – Presents as verrucous lesions that are easily misdiagnosed as warts or squamous cell carcinoma.
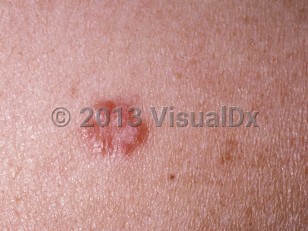
- Angiolupoid sarcoidosis – Presents as erythematous-to-violaceous papules or nodules on the lateral nose and near the medial canthus. These lesions can have surrounding telangiectasia.
- Psoriasiform sarcoidosis – Rare variant that presents with plaques that look like psoriasis.
- Tattoo sarcoidosis – Sarcoid papules and nodules can present within tattoos. This can also occur within other scars.
- Atrophic sarcoidosis – Presents as hypopigmented, shiny, or wrinkly plaques.
- Hypopigmented sarcoidosis – Characterized by hypopigmented, well-demarcated plaques.
Mortality is most commonly due to significant granulomatous disease in the lungs and heart, leading to respiratory failure, cardiac arrhythmias, and heart failure. CNS, liver, and renal diseases are also well-known causes of morbidity and mortality.
The pathogenesis of sarcoidosis is poorly understood. However, it is characterized by noncaseating epithelioid granulomas made up mostly of CD4+ helper T-cells, a predominantly Th1 type immune response, and elevated levels of interferon (IFN)-gamma and interleukin (IL)-2.
Some drugs and exposures have been associated with the development of sarcoidosis and sarcoid-like granulomatosis. Patients undergoing antiviral therapy for chronic hepatitis C – both monotherapy with IFN-alpha and combination therapy with IFN-alpha and ribavirin – have developed new-onset sarcoidosis or experienced reactivation of preexisting sarcoidosis during or shortly after treatment. The disease typically manifests as pulmonary and/or cutaneous sarcoidosis and follows a benign course, resolving spontaneously or within months after antiviral treatment is completed. More complicated multisystem cases, eg, involving the CNS, have been reported. The use of systemic corticosteroids to treat sarcoidosis in such patients should be considered with caution due to their adverse effects on viral loads. In addition, there have been an increasing number of reports of new-onset sarcoidosis manifesting in patients who are receiving anti-tumor necrosis factor (TNF)-alpha therapy (etanercept, infliximab, adalimumab), eg, for a rheumatologic diagnosis. This is paradoxical because TNF-alpha inhibitors have been used to treat sarcoidosis. Disease typically resolves with discontinuation of the drug and steroid therapy. It has been reported that exposure to moderate-to-high levels of silica increases the risk for sarcoidosis, which is of special concern for first responders (eg, firefighters). This association is more common in men due to occupational exposure.
Pediatric patient considerations: Sarcoidosis is an uncommon disease in pediatric patients and is extremely rare in children younger than 6 years. When sarcoidosis presents in children younger than 6 years, it is characterized by a triad of skin rash, uveitis, and arthritis without intrathoracic involvement. In adolescents, the cutaneous manifestations of sarcoidosis are similar to those seen in adults, with the exception of lupus pernio and erythema nodosum, which are rare in that age group.



 Patient Information for
Patient Information for